However, they can be stained in any container for which you have an. These protocols are designed for intracellular or cell surface.
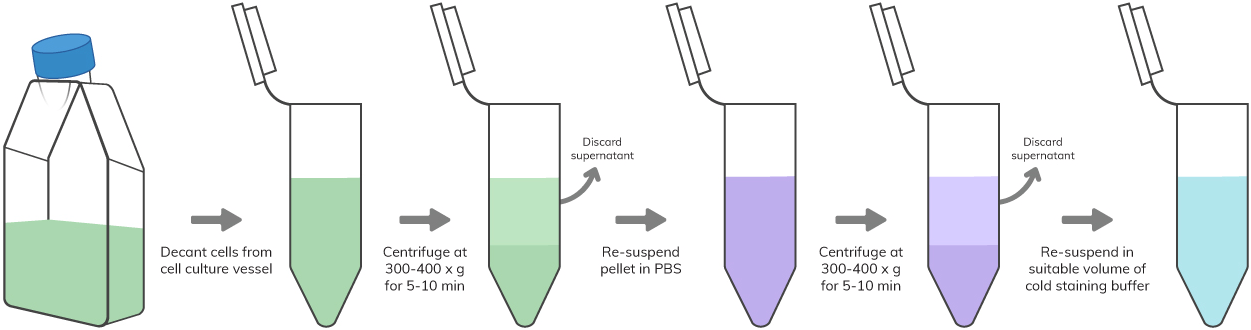
Flow Cytometry Sample Preparation
This protocol is designed for staining of cell surface proteins.

Facs flow cytometry protocol. *do not add sodium azide to buffers if you are concerned with recovering cell function e.g. The samples should be resuspended in cell staining buffer. Super bright staining buffer protocol.
Indirect staining of cells applicable when using unconjugated or biotin. The following flow cytometry staining protocols have been developed and optimized by r&d systems flow cytometry laboratory. Perform fluorescence activated cell sorting (facs), or flow cytometric analysis.
General protocols for flow cytometry. Cells are usually stained in polystyrene round bottom 12 x 75 mm2 falcon tubes. Download our membrane staining summary.
Indirect labelling requires two incubation steps, firstly with a primary antibody then with a compatible secondary antibody. Cells are usually stained in polystyrene round bottom 12 x 75 mm 2 falcon tubes. General procedure for flow cytometry using a conjugated primary antibody.
The narrow bores of the sample injection needle and tubing on a flow cytometer will be easily clogged by. Flow cytometry and facs (fluorescence activated cell sorting) are distinctly different procedures though facs is a descendant procedure based upon flow cytometry protocols. It is recommended that experimental conditions,.
For pi use 488nm laser and 585/42 bp filter. Add 3ul of diluted dye at 100x. Flow cytometry (facs) protocols include sample preparation, sample staining and data acquisition & analysis.
General procedure for flow cytometry using a primary antibody and conjugated secondary antibody. Flow cytometry » flow cytometry is the technical process that allows for the individual measurements of cell fluorescence and light scattering. Resuspend cells in 300ul of facs buffer (1x pbs + 2% bsa + 2.5mm edta).
Direct staining of cells applicable where the fluorophore is directly linked to the primary antibody. Collect and spin cells down (500 x g, 5min, 4 ° c). Indirect labeling requires two incubation steps, firstly with a primary antibody, then with a compatible secondary antibody.
The flow cytometry protocols below provide detailed procedures for the treatment and staining of cells prior to using a flow cytometer. Cell preparation (at 2x107 cells/ml) pbs4 fluorochrome conjugated antibodies Flow cytometry (facs) protocols psr the bd facscalibur™ platform allows users to perform both cell analysis and cell sorting in a single benchtop system.
Direct flow cytometry protocol general procedure for flow cytometry using a conjugated primary antibody. Single cell suspensions are required for optimal staining of samples for flow cytometry. This process is performed at rates of thousands of cells per second.
» this information can be used to individually sort or separate subpopulations of cells. However, its straightforward applicability for extracellular vesicles (evs) and mainly exosomes is hampered by several challenges, reflecting mostly the small size of these vesicles (exosomes: Indirect flow cytometry (facs) protocol general procedure for flow cytometry using a primary antibody and conjugated secondary antibody.
Advancements in cell sorting technology are contributing in a big way to the molecular science landscape. The secondary (and not the primary) antibody has the Vortex and incubate in the dark for 3min before analysis.
Optimize protocols for their particular cell type. Cell preparation for flow cytometry protocols (invitrogen ebioscience reagents) red blood cell lysis protocols using ebioscience lysis buffers (invitrogen ebioscience reagents) staining cell surface targets for flow cytometry (invitrogen ebioscience reagents) The system supports a wide variety of research and clinical applications and is complemented by a broad suite of intuitive software solutions to
Multicolor staining protocol for flow cytometry (greg a. The following flow cytometry staining protocol has been developed and optimized by r&d systems flow cytometry laboratory. The overall contributions of what is learned is.
Print this indirect flow cytometry protocol.

The Principle Of Flow Cytometry And Facs 2- Facs Fluorescence Activated Cell Sorting - Youtube

Flow Cytometry Detection Of Surface And Intracellular Antigens In Pancreas From A Single Mouse Embryo - Sciencedirect

Schematic Representation Of The Flow Cytometry Protocol Download Scientific Diagram

Flow Cytometry Protocols
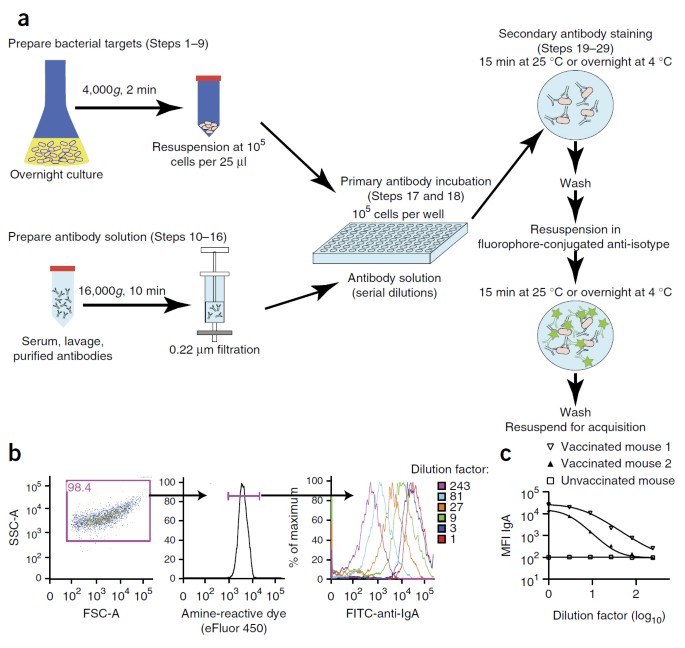
Analysis Of Bacterial-surface-specific Antibodies In Body Fluids Using Bacterial Flow Cytometry Nature Protocols
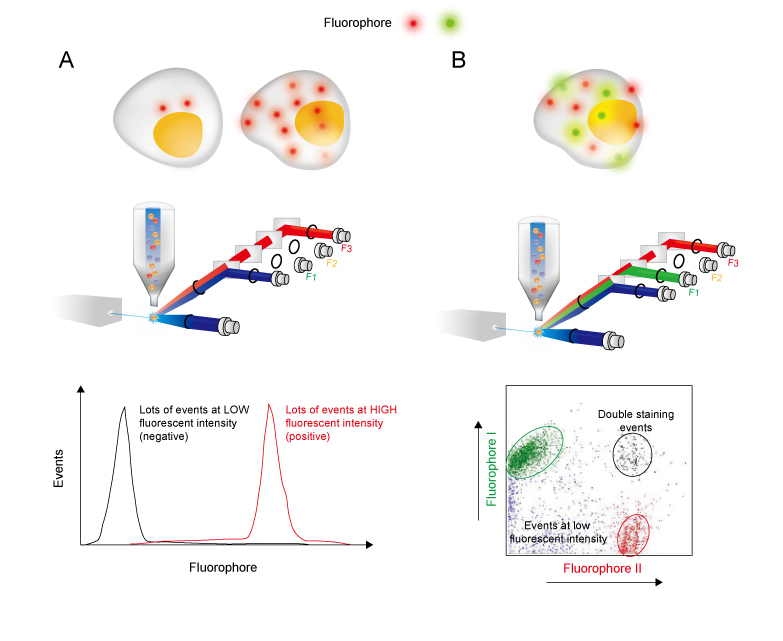
Flow Cytometry Guide - Creative Diagnostics
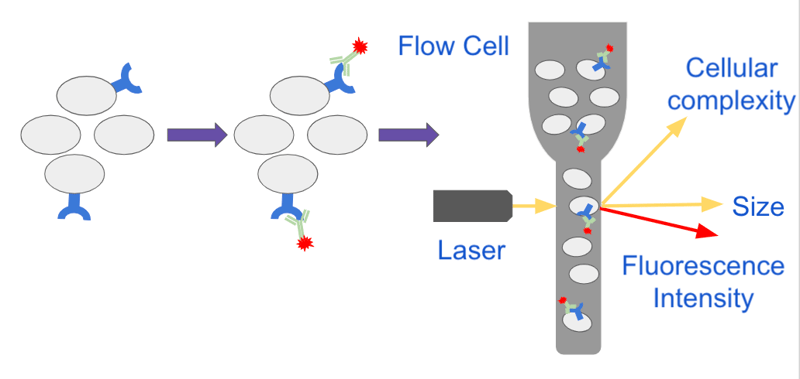
Analyzing Single Cells With Flow Cytometry
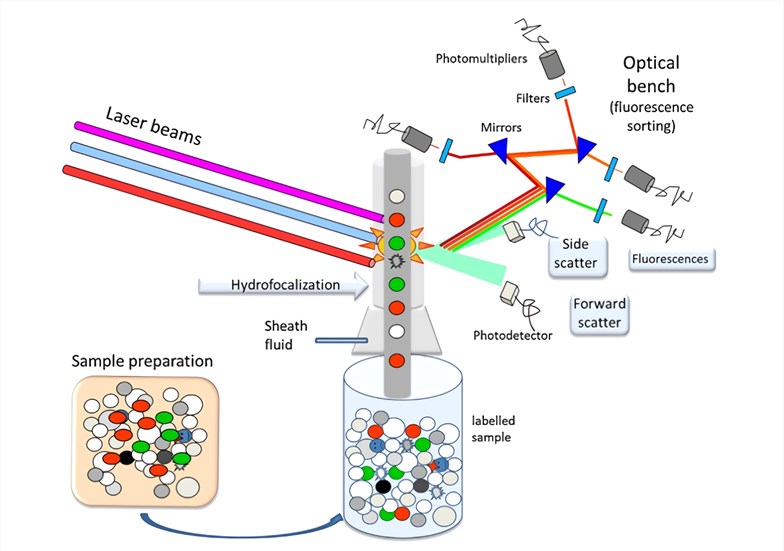
Flow Cytometry - Creative Biolabs

Protocol For Renal Cells Isolation And Macrophage Detection By Flow Download Scientific Diagram

Flow Cytometry Sample Preparation Proteintech Group
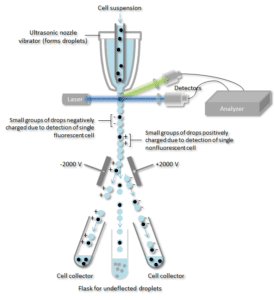
How Does Flow Cytometry Work Nanocellect

Evaluating Cytokine Production By Flow Cytometry Using Brefeldin A In Mice - Sciencedirect

Optimized Flow Cytometric Protocol For The Detection Of Functional Subsets Of Low Frequency Antigen-specific Cd4 And Cd8 T Cells - Sciencedirect

Flow Cytometry Facs Protocols Sino Biological

Flow Basics 21 The Basic Staining Protocol - Youtube

Fluorescence Activated Cell Sorting Of Live Cells Abcam

Protocol For High-throughput Compound Screening Using Flow Cytometry In Thp-1 Cells - Sciencedirect

Intranuclear Immunostaining-based Facs Protocol From Embryonic Cortical Tissue - Sciencedirect

The Principle Of Flow Cytometry And Facs 2- Facs Fluorescence Activated Cell Sorting - Youtube
Facs Flow Cytometry Protocol. There are any Facs Flow Cytometry Protocol in here.